uncertainty principal often been regarded as the most distinctive feature in which quantum mechanics differs from classical theories of the physical world.
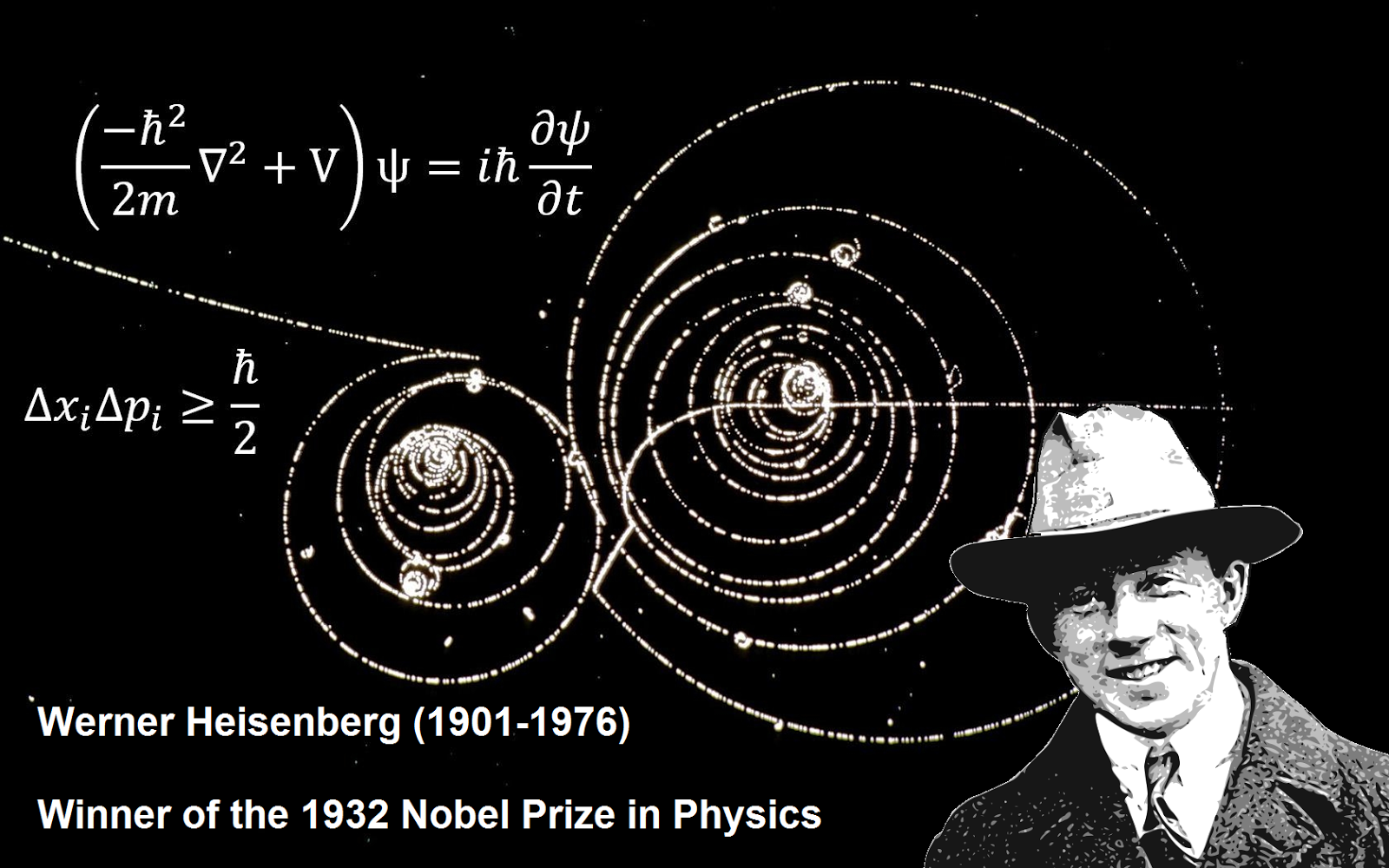
The principle that led German Physicist Werner Heisenberg to win the Nobel prize in 1932, is known as Uncertainty Principal.
The principle is that the velocity and the position of a particle of matter can never be measured simultaneously. If you go to measure one accurately, the measurement of the other is always wrong, and vice-versa; because the act of measuring itself is disturbing to the particle.
How does the uncertainty principle work?
Let's understand with an example of "ELECTRON"
Physicists know that the Electron particle revolves around the nucleus at a speed of 2192 Kilometers per second (so fast that the one round to the entire Earth can be completed in 18 seconds) but, its exact location is not known when checking the velocity of Electron. What happens is that, when you put torch-light on an Electron to know its velocity, the collision of the photon particle of light causes it to move a little, so it does not stay in its original position.In the same way, when you determine the position of an Electron at a particular moment, the photon has increased or decreased its velocity. In short, there is always “uncertainty” about one of two things i.e. Location and Speed. We do not see the significance of this problem in the everyday physical world, but it has dramatic effects on Quantum Physics; played at a level of the atom.
Heisenberg and Quantum Theory
Heisenberg was working on the impact of quantum theory; a unique and strange new way of explaining, how atoms behaved. This theory is propelled by physicists like Niels Bohr, Paul Dirac, and Erwin Schrödinger. Among its many intuitive ideas, quantum theory says that energy was not continuous but instead came in packets known as quanta and that light could be described in both; a wave and quanta.Maybe the strangest result of the uncertainty principle is what it says about the entity of vacuums. Vacuums can be defined as the absence of everything. But not so in quantum theory, It is inherent uncertainty in the amount of energy involved in quantum processes and in the time it takes for those processes to happen.
Heisenberg's uncertainty principle can also be expressed in terms of energy and time. Again, the more constrained one variable is, the less constrained the other is. It is so possible that, for very short periods of time, a quantum system's energy can be highly uncertain, so much that particles seem to come out of the vacuum.
Unknown fact
Hitler’s Nazi Germany undertook a top-secret atomic bomb-making project during World War II. Werner Heisenberg was its main facilitator. Neither Britain nor America initially knew much about the project. The two had only the slightest of suspicion.Werner Heisenberg had to move to Zurich, Switzerland in 1944, where he was to give a lecture at a convention of physicists. Knowing this, The CIA’s ancestral intelligence agency, OSS sent its secret agent to Switzerland.
The secret agent had the clear instruction to shoot him right away if he even remotely hinted at a Nazi atomic bomb project. Heisenberg, however, did not say anything about the atomic bomb thus survived.
For more interesting articles like this please check out the science section.

Post A Comment:
0 comments so far,add yours
Please do not add any spam link in the comment box